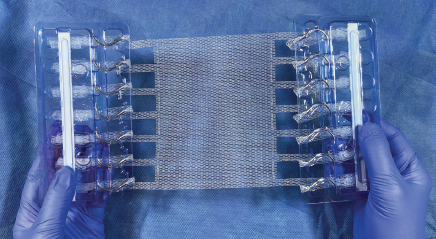
Mesh and fixation, all in one
OPTIMAL FIXATION
INTUITIVE, STRONGER ANCHORING
EXCEPTIONAL TENSION ADJUSTMENT
REDUCED TISSUE STRESS
Mesh should stay where you put it
Deep Blue is addressing the problem of failure in soft tissue surgery due to suture pull-through of tissue or mesh. T-Line® Hernia Mesh is designed to eliminate this key point of failure for conventional mesh fixation - the mesh, suture, tissue interface - and to provide superior anchor strength.
Integral suture-like extensions
More surface area than sutures
Greater fixation strength than sutures
Surgeon invented, patented
Compared to suture
T-Line Hernia Mesh extensions have:
15x more surface area for fixation (6)
275% greater fixation strength (6)
Strength of mesh extension vs traditional sutures
Greater surface area equals improved fixation strength
Similar to the roots of a tree, the T-Line Hernia Mesh extensions increase surface area, yielding greater anchor strength.
Excellent performance, innovative mesh design
T-Line Hernia Mesh is a thin, medium-weight, large-pore mesh that offers excellent bio-incorporation. This ravel-resistant mesh has a patent-pending weave that gives it exceptional performance characteristics. The T-Line Hernia Mesh is available in a wide range of sizes, all with seamless mesh extensions and integrated fixation needles.
* = mesh fibers; black arrows = fibrous capsule
In a GLP swine study, at 6 months the T-Line Hernia Mesh showed significantly greater bio-incorporation and less inflammation than the Control Mesh, as illustrated by the ubiquitous, intense pink staining of fibrous tissue capsules. In contrast, the Control Mesh was observed to have a smaller fibrous capsule with less bio-incorporation.
INDICATIONS FOR USE
Indications
T-LINE® HERNIA MESH is indicated for the reinforcement of soft tissue where weakness exists for the repair of ventral hernias performed via an open onlay or sublay approach in adults (greater than 21 years of age).
Contraindications
T-LINE® HERNIA MESH is contraindicated in infants, children, patients with known allergies to polypropylene, or in whom future growth or tissue expansion could be compromised by use of such mesh material (such as infants, children, or women who may become pregnant). Do not use T-LINE® HERNIA MESH in patients with existing infections, or with open surgical sites or fascial defects that cannot be closed. Literature reports that there may be a possibility for adhesion formation when the polypropylene mesh is placed in direct contact with the bowel or viscera. Adhesion formation with exposed mesh may cause bowel obstruction, erosion and fistula formation. Closing the peritoneum or placement of the hernia sac or omentum between the abdominal wall closure and the bowel may decrease the consequences of adhesions in the event the primary closure dehisces below the onlay and sublay mesh.
References
1. Xing L, Culbertson EJ, Wen Y, Franz MG. Early laparotomy wound failure as the mechanism for incisional hernia formation. J Surg Res 2013;182(1):e35-42. 2. Pollock AV, Evans M. Early prediction of late incisional hernias. Brit J Surg. 1989;76(9):953-954. 3. Williams JF, Kirkpatrick J, Syme GA. Force measurement in the abdominal wall. Biomed Eng 1975; 10: 181-183 4. Pott PP, Schwarz ML, Gundling R, Nowak K, Hohenberger P, Roessner ED. Mechanical properties of mesh materials used for hernia repair and soft tissue augmentation. PLoS One 2012; 7: e46978 5. Rastegarpour A, Cheung M, Vardhan M, Ibrahim MM, Butler CE, Levinson H. Surgical mesh for ventral incisional hernia repairs: Understanding mesh design. Plast Surg (Oakv). 2016;24(1):41-50. 6. Ibrahim et al. Abstract 127: A New Hernia Mesh Precisely Engineered to Prevent Hernia Recurrence. Plast Reconstr Surg Glob Open. 2018 Apr; 6(4 Suppl): 100. 7. Internal data on file. 8. GLP animal study. Data on file.
Caution: Federal law restricts this device to sale by or on the order of a physician.